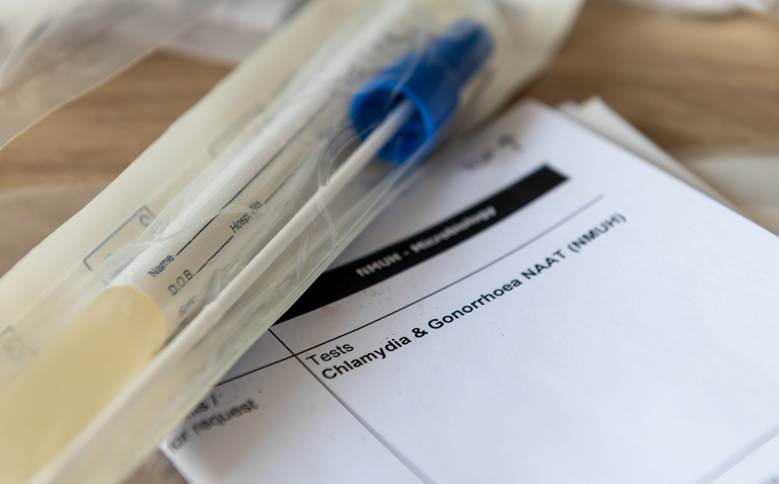
In recent years, the global health community has witnessed a concerning resurgence in sexually transmitted infections (STIs), particularly gonorrhea and chlamydia. According to the Centers for Disease Control and Prevention (CDC), these two infections remain among the most commonly reported STIs in the United States and many other developed countries. Genesis Reference Labs explains that one of the most effective strategies for combatting this rise has been the implementation of dual testing protocols—a bundled testing approach that simultaneously screens for both gonorrhea and chlamydia in a single diagnostic procedure.
This streamlined approach has not only enhanced the accuracy and efficiency of STI screening but has also delivered significant benefits in terms of public health outcomes, cost-effectiveness, and patient care. As STI laboratories continue to modernize and adapt to the growing demand for accurate, timely diagnostics, dual testing has become commonplace.
The Case for Dual Testing
Gonorrhea and chlamydia share several clinical similarities. Both are bacterial infections transmitted primarily through sexual contact, and both frequently present without symptoms—especially in women. When left untreated, they can lead to severe health consequences, including pelvic inflammatory disease (PID), infertility, ectopic pregnancy, and an increased risk of acquiring or transmitting HIV. Because the symptoms are often indistinct or entirely absent, regular testing becomes crucial for early detection and effective treatment.
What makes dual testing particularly compelling is the high co-infection rate. Studies have shown that individuals who test positive for one of these infections often test positive for the other. Dual testing therefore provides a comprehensive diagnostic picture, allowing clinicians to initiate simultaneous treatment and reducing the likelihood of untreated co-infections.
Moreover, from a workflow standpoint, dual testing eliminates the need for redundant sample collection. A single urine sample or vaginal/urethral swab can be used to test for both infections, making the process more comfortable for patients and more efficient for labs.
Bundled Testing Strategies in STI Labs
Modern laboratories are increasingly leveraging nucleic acid amplification tests (NAATs)—highly sensitive and specific molecular techniques that detect the genetic material of the pathogens responsible for gonorrhea (Neisseria gonorrhoeae) and chlamydia (Chlamydia trachomatis). NAATs can be performed on non-invasive specimens such as urine or self-collected vaginal swabs, and they are now the gold standard in STI diagnostics.
Bundled testing strategies typically involve multiplex NAAT platforms, which test for multiple pathogens simultaneously. In addition to gonorrhea and chlamydia, some panels may include testing for Trichomonas vaginalis, Mycoplasma genitalium, or even viral STIs like herpes simplex virus (HSV). However, for public health screening programs and routine clinical care, gonorrhea and chlamydia remain the core focus.
Laboratories implementing bundled testing should also adopt robust quality assurance measures. Best practices include:
- Proper specimen handling and storage to ensure test accuracy
- Validated NAAT platforms that meet regulatory standards
- Routine proficiency testing and calibration of diagnostic instruments
- Ongoing staff training and competency assessments
Additionally, clear communication with clinicians about test interpretations, especially in cases of indeterminate or low-positive results, is essential for ensuring appropriate follow-up and treatment.
Public Health Benefits of Bundled STI Testing
The public health benefits of dual testing for gonorrhea and chlamydia are substantial. Early and accurate diagnosis facilitates timely treatment, which reduces the risk of complications and onward transmission. This, in turn, helps to break the cycle of infection within communities.
Bundled testing also plays a critical role in STI surveillance, which enables public health officials to monitor trends, identify outbreaks, and allocate resources where they are most needed. With consistent use of dual testing, health departments can gather more accurate epidemiological data, which informs targeted prevention campaigns and policy decisions.
Moreover, dual testing can be cost-effective. By combining two tests into a single diagnostic event, healthcare providers can reduce the overall cost per patient screened, while increasing the likelihood of detecting infections that might otherwise go unnoticed. This is especially important in high-volume settings like community health centers, urgent care clinics, and correctional facilities, where streamlined testing protocols can improve access and reduce logistical barriers to care.
Addressing Stigma and Increasing Accessibility
One of the enduring challenges in STI prevention is the stigma associated with testing. Many individuals, especially young adults and marginalized populations, avoid testing due to fear of judgment, lack of awareness, or logistical constraints.
Bundled testing can help address this issue by normalizing comprehensive STI screening as a routine part of preventive healthcare. Educational campaigns that promote dual testing as simple, fast, and non-invasive can increase patient willingness to get tested. Additionally, the integration of dual testing into telehealth and at-home collection kits is expanding access, particularly in rural or underserved areas.
For example, some public health agencies and private companies now offer mail-in STI testing kits that allow individuals to collect samples in the privacy of their own homes and send them to a certified lab for analysis. These programs have shown high patient satisfaction and have been effective in reaching populations that might otherwise fall through the cracks.
The Future of Dual Testing in STI Care
As technology continues to evolve, dual testing protocols will likely become even more refined. Point-of-care tests with rapid turnaround times are already being developed and validated, offering the possibility of real-time diagnosis and treatment during a single clinical visit.
Furthermore, the integration of electronic health records (EHRs) with laboratory information systems (LIS) can streamline the reporting process, flag repeat infections, and support automated reminders for follow-up testing or partner notification.
To maximize the impact of dual testing, a coordinated effort is needed across healthcare providers, laboratories, public health agencies, and community organizations. By aligning strategies and investing in education, outreach, and innovation, the healthcare system can more effectively manage the burden of gonorrhea and chlamydia—and move closer to reducing STI rates overall.
Dual testing for gonorrhea and chlamydia is more than a laboratory efficiency—it is a public health imperative. Through bundled testing strategies, we can improve diagnostic accuracy, enhance patient outcomes, reduce transmission, and ultimately make STI care more accessible, inclusive, and effective. As best practices in modern STI labs continue to evolve, dual testing will remain a vital tool in the fight against these pervasive infections.